Your doctor is your trusted partner in your healthcare journey with chronic graft versus host disease (cGVHD). Please follow their guidance about starting and taking IMBRUVICA®. Once you start, it is important to take IMBRUVICA® every day as directed by your doctor to get the full benefit of your treatment.
IMBRUVICA® is an oral, once-a-day treatment for adults with cGVHD that gives you the freedom to take it with a glass of water, with or without meals, on a schedule that meets your needs. IMBRUVICA® provides the ease of oral dosing—so you can focus more on what really matters.
IMBRUVICA® tablets come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
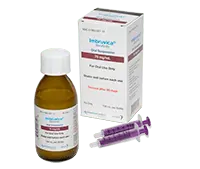
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
The bottle comes with two 3-mL oral syringes
Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
IMBRUVICA® is dosed once a day as a single 420-mg tablet, three 140-mg capsules, or 6 mL of liquid oral suspension (6 mL=420 mg). If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension.
IMBRUVICA® tablets
come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
The bottle comes with two 3-mL oral syringes
Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®

Starting IMBRUVICA®?
Get information and resources to support you along your treatment journey.*
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
For patients 1 to less than 12 years of age, talk to your doctor about dose options and the correct dose amount (based on the child's weight and height).
Helpful resources for preparing and dosing liquid IMBRUVICA®
Learn more about IMBRUVICA® oral suspension
- Glass bottle with child-resistant screw cap
- 2 reusable/washable oral dosing syringes
- Dose amount is dependent on the child’s weight, height, and age
- Take or give IMBRUVICA® exactly as your healthcare provider tells you to take it.
- Take or give IMBRUVICA® 1 time a day at about the same time each day.
IMBRUVICA® comes as capsules, tablets, and oral suspension.
-
If your healthcare provider prescribes IMBRUVICA® capsules or tablets:
- Swallow IMBRUVICA® capsules or tablets whole with a glass of water.
- Do not open, break, or chew IMBRUVICA® capsules.
- Do not cut, crush, or chew IMBRUVICA® tablets.
-
If your healthcare provider prescribes IMBRUVICA® oral suspension:
- See the detailed Instructions for Use that comes with IMBRUVICA® oral suspension for information about the correct way to take or give a dose. If you have questions about how to take or give IMBRUVICA® oral suspension, talk to your healthcare provider.
- Do not use if the carton seal is broken or missing.
- If you miss a dose of IMBRUVICA®, take or give it as soon as you remember on the same day. Take or give the next dose of IMBRUVICA® at the regular time on the next day. Do not take or give extra doses of IMBRUVICA® to make up for a missed dose.
- If you take too much IMBRUVICA®, call your healthcare provider or go to the nearest hospital emergency room right away.
Do not drink grapefruit juice
Do not eat grapefruit
Do not eat Seville oranges, often used in marmalades
These products may increase the amount of IMBRUVICA® in your blood.
Before taking any medicine, it’s important to understand its benefits and risks. Your doctor can help you understand what to expect before starting treatment with IMBRUVICA® and can address side effects if you experience them.
Your doctor could consider a dose adjustment to manage certain side effects so that you may continue to benefit from treatment, including pausing or reducing your dosage. However, in some cases, IMBRUVICA® will need to be stopped permanently.
IMBRUVICA® may cause serious side effects, including:
Bleeding problems (hemorrhage)
Infections
Heart rhythm problems (ventricular arrhythmias, atrial fibrillation, and atrial flutter), heart failure and death
High blood pressure (hypertension)
Decrease in blood cell counts
Second primary cancers
Liver problems
Tumor lysis syndrome (TLS)†
†TLS is a disorder caused by the fast breakdown of cancer cells, which can lead to kidney failure and other abnormalities.
The most common side effects of IMBRUVICA® in adults or children 1 year of age and older with cGVHD include1:
- Tiredness
- Low red blood cell count (anemia)
- Bruising
- Diarrhea
- Low platelet count
- Muscle, bone, and joint pain
- Fever
- Muscle spasms
- Mouth sores (stomatitis)
- Bleeding
- Nausea
- Stomach pain
- Pneumonia
- Headache
In the adult and pediatric cGVHD clinical trials, nearly 1 in 4 patients stopped taking IMBRUVICA® because of side effects.1
This is not a complete list of side effects. Others may occur. Tell your doctor if you think you are experiencing side effects.
The suggestions below may help with your IMBRUVICA® treatment.
To help when experiencing diarrhea2:
- Stay hydrated. Drink fluids such as water, decaffeinated tea, and clear broth
- Eat small meals often, and avoid very hot or spicy foods
- Avoid greasy foods, bran, and raw fruits and vegetables
- Avoid alcohol and drinks with caffeine
Diarrhea is a common side effect in people who take IMBRUVICA®. Drink plenty of fluids during treatment with IMBRUVICA® to help reduce your risk of losing too much fluid (dehydration) due to diarrhea. Tell your healthcare provider if you have diarrhea that does not go away.
To help reduce tiredness3:
- Balance periods of light movement with periods of rest
- Get plenty of sleep, which may include short naps
- Remain well hydrated throughout the day
- Eat a well-balanced diet
To help prevent infection4:
- Wash hands often and bathe every day
- Avoid crowds and individuals with contagious diseases
- Do not keep fresh flowers or live plants in your living space
- Do not clean up droppings from your pets; have someone else do this for you
Infection is a serious possible side effect of IMBRUVICA®. Notify a healthcare professional immediately if signs of infection (eg, fever, chills, weakness, and confusion) occur.1
See information about storing IMBRUVICA® here.
IMBRUVICA® By Your Side patient support program is not intended to provide medical advice, replace prescribed treatment plans, or provide treatment or case management services. Patients are advised to talk to their healthcare provider and treatment team about any medical decisions and concerns they may have.
References: 1. IMBRUVICA® (ibrutinib) Prescribing Information. 2. American Cancer Society. Getting Help for Diarrhea. Revised April 2020. Accessed August 6, 2025. https://www.cancer.org/




