How to take once-daily IMBRUVICA®
IMBRUVICA® is an oral, once-a-day treatment that gives you the freedom to take with a glass of water, with or without meals, on a schedule that meets your needs. IMBRUVICA® provides the ease of oral dosing—so you can focus more on what really matters.


CLL/SLL: Adults
IMBRUVICA® is dosed once a day as a single 420-mg tablet, three 140-mg capsules, or 6 mL of liquid oral suspension (6 mL=420 mg). If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension.
IMBRUVICA® tablets
come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
The bottle comes with two 3-mL oral syringes
Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
WM: Adults
IMBRUVICA® is dosed once a day as a single 420-mg tablet, three 140-mg capsules, or 6 mL of liquid oral suspension (6 mL=420 mg). If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension.
IMBRUVICA® tablets
come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
The bottle comes with two 3-mL oral syringes
Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
Previously treated cGVHD: Adults
IMBRUVICA® is dosed once a day as a single 420-mg tablet, three 140-mg capsules, or 6 mL of liquid oral suspension (6 mL=420 mg). If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension.
IMBRUVICA® tablets
come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
The bottle comes with two 3-mL oral syringes
Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
Previously treated cGVHD: Children age 12 years and older
IMBRUVICA® is dosed once a day as a single 420-mg tablet, three 140-mg capsules, or 6 mL of liquid oral suspension (6 mL=420 mg). If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension.
IMBRUVICA® tablets
come in a blister pack
Take one 420-mg IMBRUVICA® tablet by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® tablets blister pack
It contains 28 tablets (a 4-week supply)
Each tablet is identical
Blister packs are available in different tablet strengths. Contact your doctor for dosing instructions
OR
IMBRUVICA® capsules come in a bottle
Take all three 140-mg IMBRUVICA® capsules by mouth at about the same time each day with a glass of water.
Learn more about the IMBRUVICA® capsules bottle
The bottle contains a 30-day supply based on recommended dosing
Each capsule is identical
OR
IMBRUVICA® oral suspension comes in a bottle
Take 6 mL of liquid IMBRUVICA® oral suspension by mouth at about the same time each day. Make sure to drink water after swallowing the dose.
Learn more about the IMBRUVICA® oral suspension bottle
Supplied in a glass bottle with child-resistant screw cap
2 reusable/
washable oral dosing syringes Each mL of liquid oral suspension contains 70 mg of IMBRUVICA®
Tablets and capsules are not shown at actual size. Talk to your doctor about which dosing option is right for you.
Previously treated cGVHD: Children age 1 year to less than 12 years of age
For patients 1 to less than 12 years of age, talk to your doctor about dose options and the correct dose amount (based on the child's weight and height).
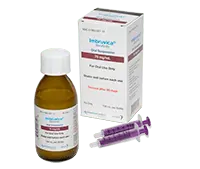
Helpful resources for preparing and dosing liquid IMBRUVICA®
Learn more about the IMBRUVICA® oral suspension bottle
Glass bottle with child-resistant screw cap
2 reusable/washable oral dosing syringes
Dose amount is dependent on the child's weight, height, and age
What you should know about the side effects of IMBRUVICA®
Before taking any medicine, it’s important to understand its benefits and risks. Your doctor can help you understand what to expect before starting treatment with IMBRUVICA® and can address side effects if you experience them.
Your doctor could consider a dose adjustment to manage certain side effects so that you may continue to benefit from treatment, including pausing or reducing your dosage. However, in some cases IMBRUVICA® will need to be stopped permanently.
Take IMBRUVICA® exactly as your doctor instructs.
The most common side effects of IMBRUVICA® in adults with B-cell malignancies (CLL/SLL and WM) include low platelet count; diarrhea; tiredness; muscle, bone, and joint pain; low white blood cell count; rash; low red blood cell count (anemia); bruising; and nausea.
The most common side effects of IMBRUVICA® in adults or children 1 year of age and older with cGVHD include tiredness; low red blood cell count (anemia); bruising; diarrhea; low platelet count; muscle, bone, and joint pain; fever; muscle spasms; mouth sores (stomatitis); bleeding; nausea; stomach pain; pneumonia; and headache.
These are not all the possible side effects of IMBRUVICA®. Please read the full Important Side Effect Information to learn about these and other risks.
Also important to know:
- Take or give IMBRUVICA® exactly as your healthcare provider tells you to take or give it.
- Take or give IMBRUVICA® 1 time a day at about the same time each day.
IMBRUVICA® comes as capsules, tablets, and oral suspension.
-
If your healthcare provider prescribes IMBRUVICA® capsules or tablets:
- Swallow IMBRUVICA® capsules or tablets whole with a glass of water.
- Do not open, break, or chew IMBRUVICA® capsules.
- Do not cut, crush, or chew IMBRUVICA® tablets.
-
If your healthcare provider prescribes IMBRUVICA® oral suspension:
- See the detailed Instructions for Use that comes with IMBRUVICA® oral suspension for information about the correct way to take or give a dose. If you have questions about how to take or give IMBRUVICA® oral suspension, talk to your healthcare provider.
- Do not use if the carton seal is broken or missing.
- If you miss a dose of IMBRUVICA®, take or give it as soon as you remember on the same day. Take or give the next dose of IMBRUVICA® at the regular time on the next day. Do not take or give extra doses of IMBRUVICA® to make up for a missed dose.
- If you take too much IMBRUVICA®, call your healthcare provider or go to the nearest hospital emergency room right away.
Call your doctor or pharmacist if you have any questions.
While taking IMBRUVICA®
- Do not drink grapefruit juice
- Do not eat grapefruit
- Do not eat Seville oranges, often used in marmalade
These products may increase the amount of IMBRUVICA® in your blood.
Storing IMBRUVICA®
- Store IMBRUVICA® capsules and tablets at room temperature between 68°F and 77°F (20°C and 25°C)
- Keep IMBRUVICA® capsules in the original container with the lid tightly closed
- Keep IMBRUVICA® tablets in the original carton
- Store IMBRUVICA® oral suspension bottle between 36°F and 77°F (2°C and 25°C). Do not freeze
- Use IMBRUVICA® oral suspension within 60 days after first opening the bottle. Throw away (discard) any unused portion 60 days after opening
Keep IMBRUVICA® and all medicines out of the sight and reach of children.
Tell your doctor about any other medications you are taking, including prescriptions or over-the-counter medications, vitamins, and herbal supplements. Taking IMBRUVICA® with certain other medicines may affect how IMBRUVICA® works and can cause side effects.
Some ways to stay on track with your treatment2
It’s important to take your medicines exactly as directed by your doctor and creating a routine may help with your IMBRUVICA® treatment. Here are some ways to help you stay on course:
References: 1. IMBRUVICA® (ibrutinib) Prescribing Information. 2. MedlinePlus. Taking medicine at home: create a routine. Accessed March 14, 2024. https://medlineplus.gov/ency/patientinstructions/000613.htm




