After failure of systemic therapy, children with cGVHD have another option
IMBRUVICA® is the first and only FDA-approved chronic graft versus host disease (cGVHD) treatment for kids 1 year and older after failure of one or more lines of systemic therapy.1
When prior systemic therapies haven’t worked for your child, you have a different option for continuing treatment. Because IMBRUVICA® is a BTKi, it works differently than other treatments.1 IMBRUVICA® is available in a liquid form for children, with once-a-day dosing.
For the first time ever, an FDA-approved treatment is available in a liquid form for children 1 year to less than 12 years of age who have previously treated cGVHD.*
*As of August 2022.
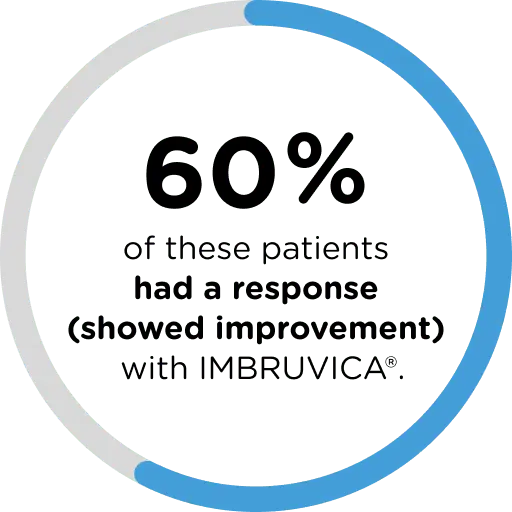
Shown to be effective in a clinical trial1
IMBRUVICA® was studied in a 25-week clinical trial of 47 previously treated cGVHD patients, ranging in age from 1 to less than 22 years of age. By week 25 of treatment:
Understanding side effects1
IMBRUVICA® may cause serious side effects, including:
- Bleeding problems (hemorrhage)
- Infections
- Heart rhythm problems (ventricular arrhythmias, atrial fibrillation, and atrial flutter)
- Heart failure and death
- High blood pressure (hypertension)
- Decrease in blood cell counts
- Second primary cancers
- Tumor lysis syndrome (TLS)†
†TLS is a disorder caused by the fast breakdown of cancer cells, which can lead to kidney failure and other abnormalities.
The most common side effects of IMBRUVICA® in the clinical trial of children and young adults ages 1 to less than 22 years with cGVHD included:
- Decrease in red blood cells
- Muscle and bone pain
- Fever
- Diarrhea
- Pneumonia
- Abdominal pain
- Mouth sores (stomatitis)
- Decrease in platelet count
- Headache
This is not a complete list of side effects. Others may occur. Tell your doctor if you think your child is experiencing side effects.
Diarrhea can be an uncomfortable side effect for patients taking IMBRUVICA®. Make sure children drink plenty of fluids during treatment to reduce the risk of losing too much fluid (dehydration). Diarrhea can also be a sign that cGVHD is getting worse. Be sure to contact the doctor right away if children develop diarrhea.
Tips to help with diarrhea include2:
- Drinking clear fluids such as water or broth
- Eating small meals often, and avoiding spicy food
- Avoiding greasy food, raw fruits and vegetables, and caffeine
Infection is a serious possible side effect of IMBRUVICA®. Contact the doctor immediately if signs of infection such as fever, chills, weakness, or confusion occur.1
Tips to help prevent infection include3:
- Make sure children wash their hands often, and bathe every day
- Keep children away from crowds or people with contagious diseases
- Don’t keep live plants or flowers in a child’s bedroom
- Do not let children come into contact with pet droppings
IMBRUVICA® dosing for children1
How should I take IMBRUVICA®?
- Take IMBRUVICA® exactly as your healthcare provider tells you to take it.
- Take IMBRUVICA® 1 time a day at about the same time each day.
IMBRUVICA® comes as capsules, tablets, and oral suspension.
- If your healthcare provider prescribes IMBRUVICA® capsules or tablets:
- Swallow IMBRUVICA® capsules or tablets whole with a glass of water.
- Do not open, break, or chew IMBRUVICA® capsules.
- Do not cut, crush, or chew IMBRUVICA® tablets.
- If your healthcare provider prescribes IMBRUVICA® oral suspension:
- See the detailed Instructions for Use that comes with IMBRUVICA® oral suspension for information about the correct way to give a dose to your child. If you have questions about how to give IMBRUVICA® oral suspension, talk to your healthcare provider.
- Do not use if the carton seal is broken or missing.
- If you miss a dose of IMBRUVICA® take it as soon as you remember on the same day. Take your next dose of IMBRUVICA® at your regular time on the next day. Do not take extra doses of IMBRUVICA® to make up for a missed dose.
- If you take too much IMBRUVICA® call your healthcare provider or go to the nearest hospital emergency room right away.
Giving IMBRUVICA® to children 1 year and older.
If your doctor prescribes liquid IMBRUVICA® oral suspension, refer to the detailed Instructions for Use that comes with IMBRUVICA® oral suspension. It provides information about the correct preparation and dosing of this liquid medicine. If you have questions about how to give IMBRUVICA® oral suspension, talk to your doctor.
Read the Instructions for Use included with IMBRUVICA® Important Product Information before giving IMBRUVICA® to a child, and each time you get a refill. There may be new information.
For more information about cGVHD and IMBRUVICA® oral suspension, download the Parents and Caregivers Guide.
Ways to help plan the child’s IMBRUVICA® routine4
It’s important that children take their medicine exactly as directed by their doctor. IMBRUVICA® should be taken at about the same time each day. Creating a routine can help you remember, and help children get the most benefit from their treatment.

Discover personalized one-on-one support
IMBRUVICA® By Your Side is here to help enrolled pediatric patients with cGVHD and their caregivers by providing one-on-one support and treatment-related resources.
IMBRUVICA® By Your Side patient support program is not intended to provide medical advice, replace prescribed treatment plans, or provide treatment or case management services. Patients are advised to talk to their healthcare provider and treatment team about any medical decisions and concerns they may have.
References: 1. IMBRUVICA® (ibrutinib) Prescribing Information. 2. American Cancer Society. Getting help for diarrhea. Revised April 2020. Accessed May 9, 2023. https://www.cancer.org/content/dam/cancer-org/cancer-control/en/booklets-flyers/getting-help-for-diarrhea.pdf 3. American Cancer Society. Watching for and preventing infections. Accessed May 9, 2023. https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/infections/preventing-infections-in-people-with-cancer.html 4. MedlinePlus. Taking medicine at home: create a routine. Accessed August 18, 2023. https://medlineplus.gov/ency/patientinstructions/000613.htm



